

ii) Cs has larger atomic radius because as we move from Li to Cs. Note: Atomic radius gives the distance between the center of the nucleus and the outermost electrons/valence electron. i) Ca has larger atomic radius because as we move from Mg to Ca, the atomic radius increases. Due to this small size, it is very difficult to measure the atomic radius.

Atomic radius is similar to the radius of a circle.
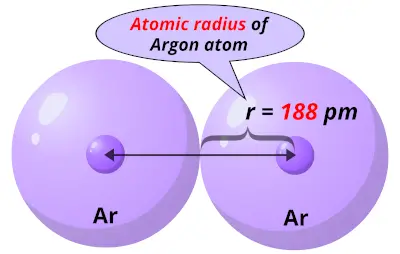
We define the atomic radius of a chemical element as: The mean or typical distance from the centre of the nucleus to the boundary of the surrounding shells of electrons. Atomic radius is one half the distance between two adjacent atoms in a molecule.Ītomic radius is usually measured in angstrom units as it is very small in size. Atomic radius or Atomic Radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. In general, atomic radius decreases across a period and increases. Main article: Atomic radius The atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. Atomic radius can be measured by either the X-ray diffraction method or some other spectroscopic methods. The element having the largest atomic radius is : a Mg b Na c K d. Atomic radius is the distance from the atoms nucleus to the outer edge of the electron cloud. Complete step by step answer -The atomic radius of an atom is defined as the distance from the center of the nucleus to the outermost shell of the atom containing electrons i.e, the valence shell. Hint: Atomic radius is the distance between the center of the nucleus and the valence shell. The atomic numbers of the elements Na, Mg, K and Ca are 11, 12, 19 and 20 respectively.


 0 kommentar(er)
0 kommentar(er)
